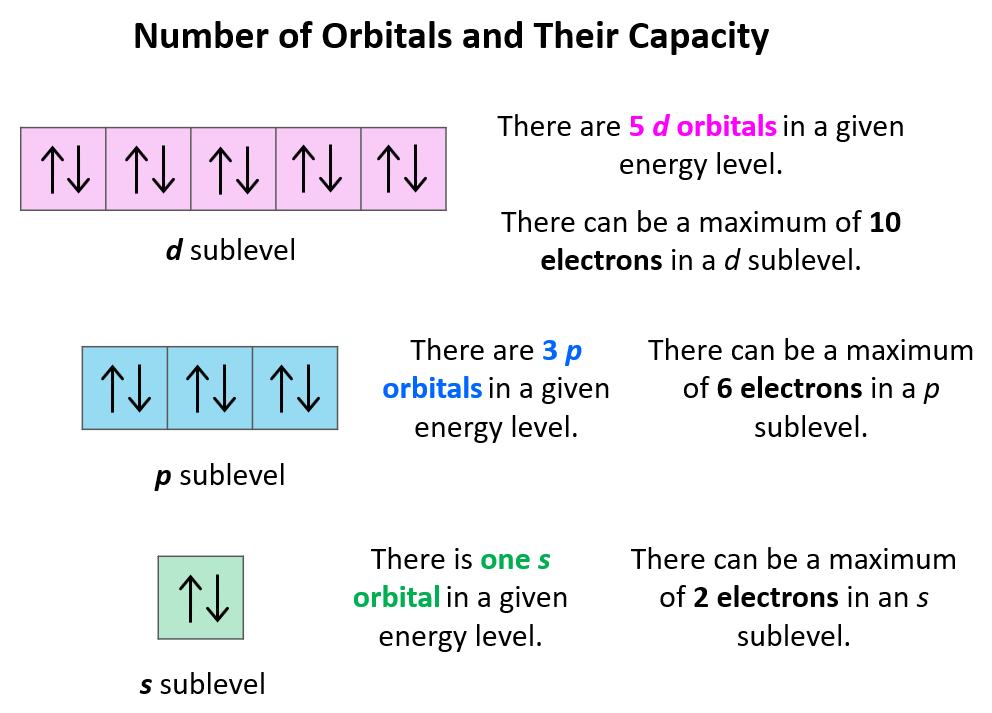
Who Else Wants Info About Do Electrons Rotate Or Revolve

Class Opener Tuesday 1)Use A Venn Diagram To Compare And Contrast The
Electrons
1. A Whimsical Dive into the Quantum Realm
Alright, let's talk about electrons. These tiny particles, the workhorses of atoms, get a lot of buzz. But when we try to picture them, things get a little...weird. We often hear about electrons "orbiting" or "spinning," but what do those words really mean in the strange world of quantum mechanics? Its not quite like the Earth revolving around the Sun, or a top spinning on a table. Buckle up, because we're about to embark on a journey into the miniature universe!
First off, let's ditch the image of tiny planets neatly circling the nucleus. That's a very outdated, and frankly, misleading picture. In reality, electrons exist in probability clouds called orbitals. Think of it like this: you can't pinpoint exactly where an electron is at any given moment. Instead, you can only say where it's likely to be found. It's like knowing a friend is usually at the coffee shop between 2 PM and 4 PM, but you can't guarantee they'll be there at 2:17 PM precisely.
Now, about that "spin." The term “electron spin” is actually a bit of a misnomer. It doesn't mean the electron is physically rotating on an axis like a little basketball. Instead, spin is an intrinsic form of angular momentum, a fundamental property of the electron, like its charge or mass. Its something electrons just have, and it's quantized, meaning it can only exist in specific values: either "spin up" or "spin down."
Imagine you have a magical compass needle that always points either directly up or directly down, regardless of how you try to tilt or spin it. That's kind of like electron spin. It interacts with magnetic fields, and it's crucial for understanding how atoms bond and form molecules. So, "spin" is more of a descriptive label than a literal rotation.

What Should Be The Direction Of Moving Electron In
So, Do Electrons Revolve? A Slightly Better Analogy, But Still...
2. Orbital Shenanigans and Quantum Leaps
When we say electrons "revolve," we're usually referring to their movement around the nucleus within those aforementioned probability clouds. Even this image can be a bit misleading though! Electrons don't follow neat, predictable paths. Instead, they exist as wave functions spread out over the orbital. They aren't tracing out paths, so really, revolving in the classical sense isn't happening either.
Think of each orbital as a specific energy level. Electrons can jump between these energy levels by absorbing or emitting energy. This is known as a quantum leap. It's like climbing up or down a staircase — you can only stand on specific steps, not in between them. When an electron absorbs energy, it jumps to a higher energy level (a higher step). When it falls back down, it releases energy in the form of light (a photon).
These quantum leaps are the basis for many technologies, like lasers and fluorescent lights. The specific colors of light emitted by different elements are determined by the energy differences between their electron orbitals. It's a beautiful, intricate dance of energy and probability.
Therefore, using the term revolve implies a defined trajectory or path, which isnt accurate for electron behavior. While it's a convenient analogy for understanding the general idea of electrons being associated with the nucleus, it falls short of describing the true quantum mechanical nature of their existence.
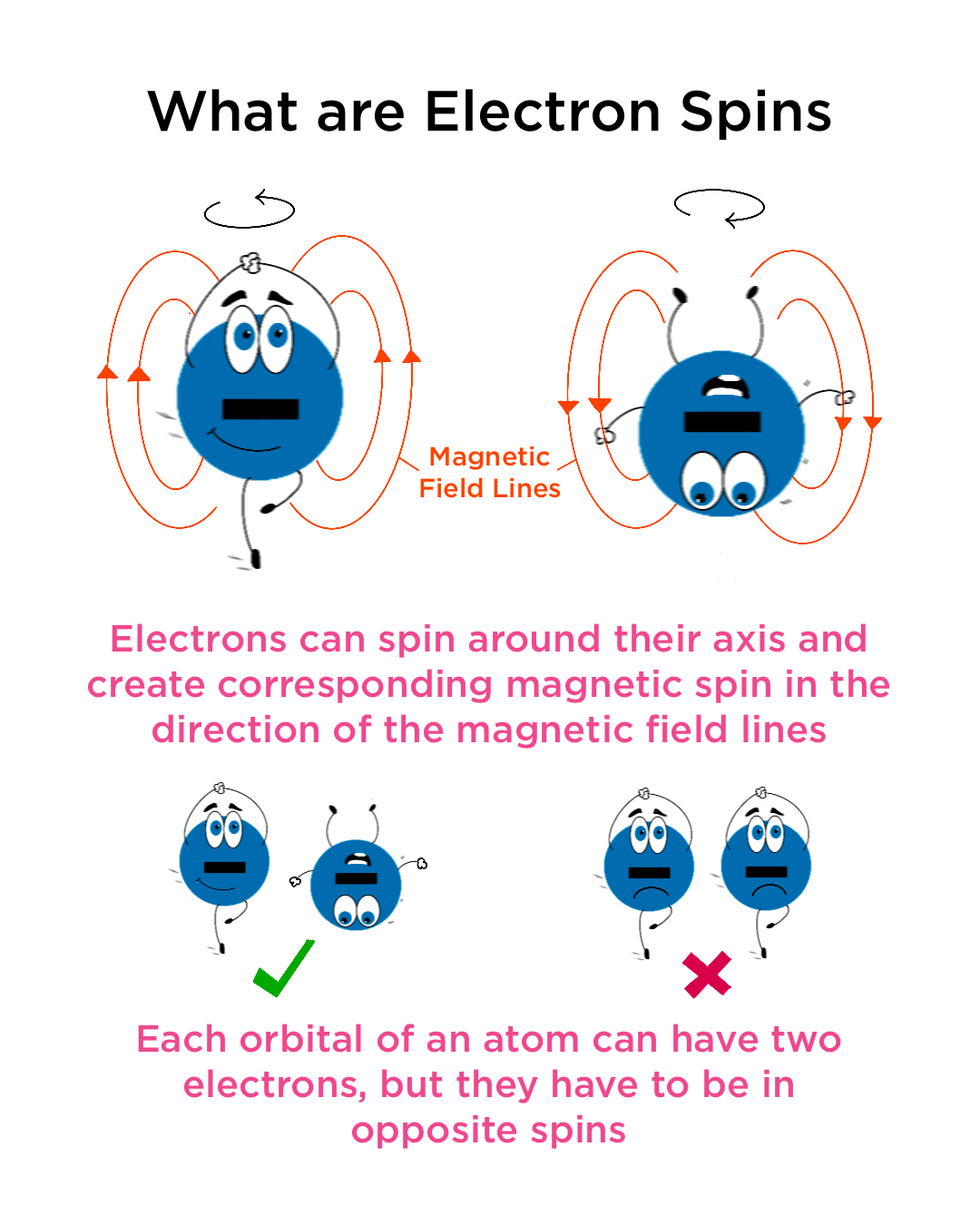
Why Does Any Of This Matter? (Besides Being Cool, Of Course)
3. The Practical Implications of Quantum Weirdness
You might be thinking, "Okay, this is interesting, but what does it all mean for me?" Well, understanding electron behavior is fundamental to understanding chemistry, materials science, and a whole host of technologies. From the semiconductors in your computer to the batteries in your phone, everything relies on the principles of quantum mechanics and how electrons behave within atoms and molecules.
Consider the properties of different materials. Why is copper a good conductor of electricity while rubber is an insulator? The answer lies in the arrangement of electrons in their atoms and how easily they can move between them. In copper, electrons are relatively free to roam, allowing electricity to flow. In rubber, electrons are tightly bound, preventing the flow of electricity.
Furthermore, the development of new materials with specific properties, such as superconductors or high-strength alloys, requires a deep understanding of electron interactions at the atomic level. Scientists are constantly pushing the boundaries of what's possible by manipulating electron behavior to create materials with unprecedented capabilities.
Even medical imaging techniques, like MRI, rely on the properties of electron spin. By manipulating the spins of atomic nuclei with magnetic fields, doctors can create detailed images of the human body. So, while the quantum world might seem abstract and detached from everyday life, it's actually deeply intertwined with the technologies and innovations that shape our modern world.

The Ongoing Quest for Understanding
4. Quantum Mechanics
It's important to remember that our understanding of electrons and quantum mechanics is still evolving. Scientists are constantly developing new theories and experiments to probe the mysteries of the subatomic world. There are still many unanswered questions, and new discoveries are being made all the time.
For example, researchers are actively exploring the potential of quantum computing, which harnesses the principles of quantum mechanics to perform calculations that are impossible for classical computers. Quantum computers could revolutionize fields like medicine, materials science, and artificial intelligence.
The study of electron behavior also has implications for our understanding of the universe itself. By studying the properties of fundamental particles, scientists can gain insights into the origins of the universe and the nature of dark matter and dark energy. It's a quest to unravel the deepest secrets of reality.
So, while we may not have all the answers about electrons and their peculiar behavior, the journey of discovery is far from over. The more we learn about the quantum world, the more we understand about ourselves and the universe we inhabit. Isn't that a thought worth pondering over a cup of coffee?
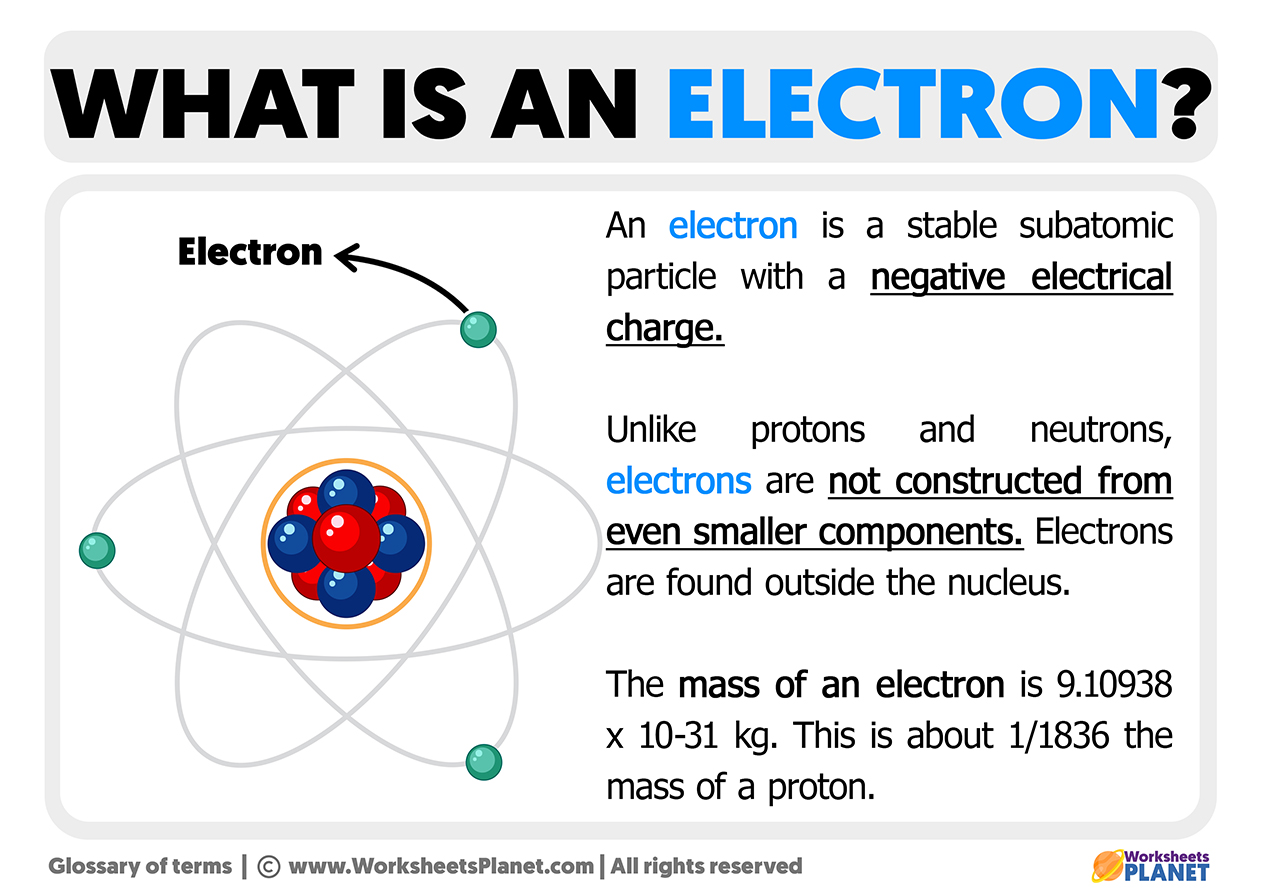
FAQ
5. Quick Answers to Common Queries
Q: Are electrons really particles or waves?A: That's the million-dollar question! Electrons exhibit wave-particle duality, meaning they can behave as both particles and waves depending on how you observe them. Spooky, right?
Q: If electrons don't really spin, why do we call it "spin"?A: "Spin" is just a convenient name for an intrinsic property of electrons. It's easier to visualize than "intrinsic angular momentum," even if it's not a perfect analogy.
Q: Will I ever need to know any of this stuff in real life?A: Maybe not directly, but a basic understanding of how electrons behave can help you appreciate the technologies that surround you every day! Plus, it's just plain cool to think about the mysteries of the quantum world.
Q: So, can I say electrons "rotate" or "revolve"?A: For a simple explanation, it's ok to use these terms to give a general idea of electron behaviour, but not accurate. In scientific contexts, it's best to avoid these terms altogether and stick to concepts like orbitals, energy levels, and spin. It's a bit more complex, but also much more accurate!